An Atoms Atomic Mass Is Best Described as
Number of neutrons 25 - 12 13. The atomic mass of an element is defined as the sum of the atomic number aka.
Atomic mass is the mass of a single atom approximately equal to the mass number of the atom.

. The atomic mass unit is also called the dalton Da after English. Which of the following best describes the atomic number of an atom. Electrons contribute so little mass that they arent counted.
The protons it contains. Protons and neutrons it contains. This small change is due to the binding energy mass loss.
An atoms atomic mass is best described as the mass of. Atomic mass the quantity of matter contained in an atom of an element. The mass of an atom can be accounted for by the sum of the mass of protons and neutrons which is almost equal to the atomic mass.
A protons it contains b neutrons it contains c electrons in the outermost shell d protons and neutrons it contains The nerve cell is specialized to use properties exhibited by all cells including. View the full answer. If this chemical cannot be separated into other chemicals then it.
An electron that moves into the atoms nucleus. According to Rutherford model of the atom. The atomic mass of an element can be described as the total mass of one atom of the given element.
2 Major space in an atom is empty. A researcher uses several procedures to separate a rock sample into different chemicals. The neutrons it containsC.
Atomic number also called proton number refers to the. What is the change in atomic mass number when an atom emits an alpha particle. In other words an atoms atomic mass is defined as the weighted average over the abundance of its isotopes.
A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom. A the number of protons in the atom B the number of electrons in the atom C the number of neutrons in the atom D the number of protons electrons and neutrons in the atom E the net electrical charge of the atom. An atoms atomic mass is best described as the mass of-its protons-the neutrons it contains-electrons in outermost shell-protons and neutrons-protons and electrons.
The neutrons it contains. An atom with an atomic number of 12 and a mass number of 25 can best be described by which of these. The total number of protons and neutrons in the nucleus.
An atoms atomic mass is best described as the mass of protons and neutrons it contains The characteristic way in which atoms of an element react is most related to the. Of the mass of a carbon-12 atom in its ground state. Mass number is the sum of number of protons and number of neutrons.
3 Atoms nucleus is surrounded by negatively charged particles called electrons. Eachhas a nominal mass of 1. Atomic number 12.
April 29 2021 thanh. Thus an atom contains 12 protons and 13. So the mass of hydrogen with one protonand no neutrons in its nucleus has a mass of 1.
Electrons in outermost shellD. An atoms atomic number is best described as the number of. 11 An atoms atomic mass is best described as the mass of A the protons it contains B the neutrons it contains.
An element with a mass number of 11 and an atomic number of 5 has how many neutrons. Atomic mass refers to the sum of number of neutrons and protons Option D present in the nucleus. Mass number Number of protons Number of neutrons 2 We are given.
Isotope 3 H refers to the isotope tritium that con. The atomic mass of an element is defined as the weighted average mass of that elements. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom 1992646547 1023 gram which is assigned an atomic mass of 12 units.
Mass number 25. Its unit is called the unified atomic mass unit and is denoted by the symbol u. For example while writing the electronic configuration.
According to the new current Quantum atomic model An orbital is a region of space in an atom where the probability of finding electrons is maximum. A mass of 50 grams of one chemical is produced. An atoms atomic mass is best described as the mass ofA.
A Separation of the fluid environments inside and outside the cell b Active transport of Na out. 1 amu 166 1024 g. Number of protons Number of electrons 12.
According to Bohrs atomic model Na atomic number 11 K shell 2 L shell 8 M shell 1 But according to the current model of an atom. The number of protons and the number of neutrons in the elements atoms. AAn atom that contains 12 protons and 13 neutronsBAn atom that contains 13 protons and 12 neutronsCAn atom that contains 12 protons and 25 neutronsDAn atom that contains 25 protons and 12 neutrons.
Atomic weight is a weighted average of the mass of all the atoms of an element based on the abundance of isotopes. Electrons in the outermost shell. 1 Atoms have their charge concentrated in a very small nucleus.
It is close to the mass number of an element sum of number of protons and neutrons To understand more clearly watch this video. Huge stadium with a positively charged marble at the center. Which statement best describes the relationship between masses of subatomic particles.
An atoms atomic mass is best described as the mass of The atomic mass of 5626fe is 55934939 u and the atomic mass of 5627co is 55939847 u. 4 An atom is electrically neutral. It can be best defined as.
This equation is very useful for finding. In this scale 1 atomic mass unit amu corresponds to 1660539040 1024 gram. Protons and electrons it contains.

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets

The Number Of Protons In The Nucleus Of An Atom Is Its Atomic Number Z It Is The Defining Trait Of An Element Its Value Dete Mass Number Atomic Number Atom

How To Calculate Atomic Mass Teaching Chemistry Relative Atomic Mass Chemistry Worksheets

Bohr Atomic Models Worksheet Answers Atom Physics Worksheet Chemistry Notes Chemistry Education Teaching Chemistry

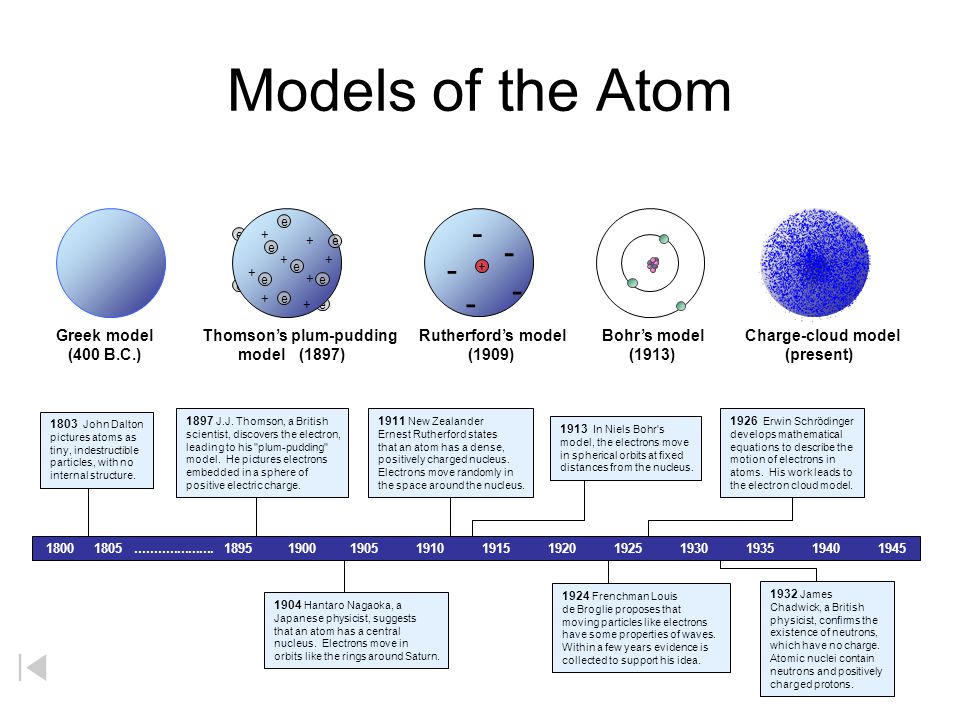
Atomic Models And Their Innovations Atomicmodel Atomicmodels Daltonmodel Thomsonmodel Rutherfordmodel Bohrmod Chemistry Lessons Atom Chemistry Classroom

This Is One Of My Best Selling Products This Lesson Introduces Atoms Provides Some History With Their Discovery Covers Atomic Lesson Atom Teaching Science

Pin By Makaylah Randolph On Science Chemistry Education Atomic Mass Unit Types Of Psychology

The Atom Chemistry Is My Jam Chemistry Classroom Atom Electron Configuration

Atoms Worksheet Teaching Chemistry Chemistry Classroom Chemistry Lessons

Atomic Structure Worksheet Chemistry Classroom Teaching Chemistry Chemistry Worksheets

Said Core Particles Google Otsing Atomic Structure Ernest Rutherford Atomic Theory

Atoms And The Periodic Table Activities Game For Learning Atomic Structure Middle School Science Classroom Chemistry Activities Chemistry Classroom

Atomic Structure Worksheet Teaching Chemistry Chemistry Lessons Chemistry Classroom

Chem 105 Activity Two Atom And Atomic Structure 5th Grade Science Science Lesson Activities Chemistry Education

The Atom Chemistry Is My Jam Protons Electron Configuration Atom

Have Your Students Practice Identifying Atomic Number Atomic Mass Number Protons Neutrons And Ele Teaching Chemistry Life Science Lessons Chemistry Lessons



Comments
Post a Comment